Enhancing electrical stability of plastic electronics devices
8 Dec 2014. NUS scientists in collaboration with Solvay OLED/ Plextronics scientists have identified the mechanism and resolved the undesirable conductivity fading phenomenon that occurs in the conducting polymer layer when plastic electronic devices are subjected to large current densities. This reveals a new insight into the mechanisms for electrochemical stability of organic semiconductors under operation conditions and suggests new strategies for their further stabilisation. The work has been published in Chemistry of Materials (Dagmawi Belaineh et ",A High-Performance p-Doped Conducting Polymer Blend Based on Sulfonated Polyalkoxythiophene and Poly(4-hydroxystyrene)", Chem. Mater., 26 (2014) 4724, DOI: 10.1021/cm5012229).
Organic semiconductors are carbon-based organic materials that have useful semiconductor properties, such as light emission, photocurrent generation and conductivity switching. In addition these materials can often be processed by inexpensive methods such as liquid coating and printing methods, which makes possible the fabrication and deployment of high performance organic light-emitting diodes, solar cells and transistor circuits on large and/or flexible areas. However a challenge is that the p-doped conducting polymer layer often used as hole injection or hole extraction layer, or as interconnects and electrodes in electrochromic devices, batteries and capacitors, can fail when large current densities are sustained through them over a period of time.
Now scientists working on a new p-doped conducting polymer material developed by Solvay OLED/ Plextronics, sulfonate poly(thiophene-3-(2-(2-methoxyethoxy)ethoxy)-2,5-diyl): poly(4-hydroxystyrene) ( S-P3MEET:PHOST), commercially sold as XA-3551, have found that it is surprisingly more resilient to conductivity fading than other p-doped conducting polymers such as the standard poly(3,4-ethylenedioxythiophene): poly(styrenesulfonic acid) which is in widespread use. The greatly enhanced electrical stability was found to arise from the shutdown of a coupled ion-electron conduction path by the ultralow ionic conductivity of the poly(4-hydroxystyrene) matrix polymer and a serendipitous solid-state reaction in the polymer film that removes acidic hydrogen atoms from the sulfonic acid groups. These two mechanisms together deprive the material of the source of ions that trigger the undesirable change in the doping level of the film when a current is passed through it. This work reveals that while electronic conductivity is desirable, ionic conductivity is not desirable in doped conducting polymers. Suppression of the ionic conductivity is central to improving the electrical stability of its doping level and hence the stability of devices incorporating these materials.
"This work originated from a research contract agreement between our group at NUS and the Plextronics team to study the hole-injection materials they have developed, leveraging on our expertise on organic semiconductors, devices and degradation studies. We are delighted that the project has grown and developed unexpected general insights into the behavior of organic semiconductors. The findings here reveal important design considerations for future high-stability doped conducting polymers," says Dr Rachael Png, leader of the NUS group performing this study in the laboratory of Prof Peter Ho.
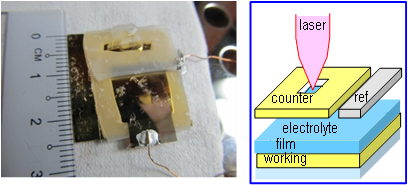
Photo and schematic structure of the home-built thin-layer encapsulated cell suitable for Raman spectroelectrochemical experiments of conjugated polymer thin films while under an electrochemical potential applied in a three-electrode configuration

